James Chadwick (1891-1974)

|
Current Atomic Model 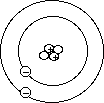
|
- Chadwick in collaboration with Rutheford realized that the atomic
mass of most elements was about double the number of protons. For example,
helium has an atomic mass of about 4 and only 2 protons. Electrons have minimal
relative mass, so they postulated that a neutral particle may exist.
- Irene (Marie Curie's daughter) and Frederic Joliot-Curie were conducting
many radiation experiments during this time. However, they were not searching
for neutrons. Chadwick realized that some of their experiments might be used
to find the elusive neutron, so he repeated their experiments with this goal
in mind.
- In 1932, he found definitive evidence for the existence of a neutral
particle that had roughly the same mass as a proton. He discovered the
neutron.


