Activation Energy
- As mentioned before, to get a reaction to happen bonds usually
have to be broken first. This takes some energy, usually in the form of heat
(fast moving molecules or atoms colliding) or light.
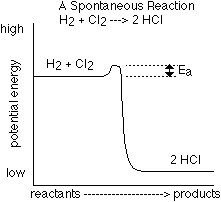
- The energy needed to break the initial bonds of the reactants is called
the Activation Energy and is abbreviated Ea. Below is a picture
of two different reactions and how the chemical potential energy of the substances
changes during the reaction.

- Notice that the endothermic reaction needs a continual input of energy to
continue reacting. However, the reaction between H2
and O2
gives off so much energy that it can supply the left over reactants with the
activation energy needed to form water and complete the reaction. Once this
reaction is started with a spark or a flame it continues until there are no
more reactants.
- Exothermic reactions with a very low activation energy will occur spontaneously,
and one's that tend to give off a lot of energy tend to be very reactive or
even explosive.