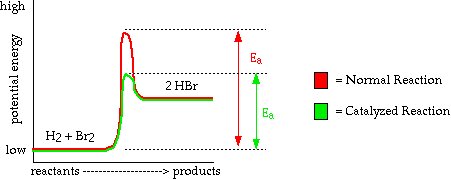
- Most of the enzymes in your body are catalysts. Many of the chemical reactions that are necessary for life to occur run too slowly without being catalyzed by an enzyme. Without catalysts we could not exist.
- The catalytic converter that is part of all modern car exhaust systems. This turns many of the pollutants (primarily hydrocarbon (C-H) fragments and carbon monoxide (CO) in the exhaust) into carbon dioxide (CO2) and water (H2O).
- When you generated oxygen from hydrogen peroxide earlier this year you
used a Manganese metal catalyst.
2 H2O2 ---> 2 H2O + O2
Notice that the MnO2 is not written as part of this reaction. That is because it is not consumed during the reaction. It can be reused over and over again. - Chlorine atoms from CFCs (chloroflourocarbons) catalyze the breakdown of ozone into oxygen: O + O3 ---> 2 O2