Phase Change and Chemical Potential Energy
- There is chemical potential energy in the weak van der Waals bonds
as well as in the strong ionic and covalent bonds.
- Because the van der Waals bond/attraction is so much weaker there is much
less chemical potential possible here, but it is a major factor in the energy
of phase changes.
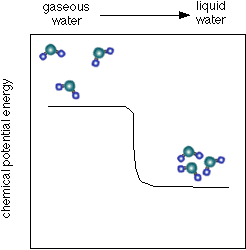
- As you can see below, the chemical potential energy of water is less than
that of water vapor (or steam) which helps to explain why steam burns can
be so severe. Not only is the steam hot, but it tends to condense into a liquid
when it touches something. When forming a liquid the chemical potential energy
that is released when the van der Waals bonds form can add substantial heat
energy to the process of heat transfer from the water molecules to whatever
they are condensing on.
- See a diagram of the energy below:
- A process that releases chemical potential energy as heat is called exothermic.
- A process that absorbs heat to increase chemical potential energy is called
endothermic.
- Whenever a change of state occurs energy is either absorbed or released
- An exothermic process releases heat (converting
some of its chemical potential energy to heat energy).
- An endothermic process absorbs heat (converting some of
the heat energy into chemical potential energy.)
- Below is a table of common phase changes and their associated energy
|
Process |
Name of phase change |
Energy flow |
|
solid -> liquid |
|
|
|
liquid -> solid |
|
|
|
liquid -> gas |
|
|
|
gas -> liquid |
|
|
|
solid -> gas |
|
|
|
gas -> solid |
|
|
- In endothermic phase changes the energy is used to overcome the weak intermolecular
bonds.
- In exothermic phase changes some chemical potential energy is released as
heat energy when the weak intermolecular bonds form.