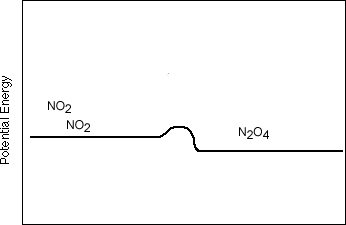
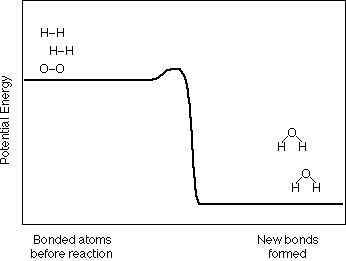
2 H2 + O2  2 H2O
2 H2O

In other words, why is it less likely that water breaks up to form hydrogen and oxygen than it is for hydrogen and oxygen to form water?
2 H2 + O2  2 H2O
2 H2O
to show that it is mostly water that is formed.
to show that it is mostly water that is formed.
2 H2 + O2  2 H2O
2 H2O