The size of a molecule can affect the London dispersion
force between two molecules. The more surface area there is on a molecule
the greater chance there will be at least one instantaneous dipole at any
particular moment. Therefore, the greater the surface area (generally this
means the bigger the molecule) the stronger the attraction between two molecules
of this type due to London dispersion forces
| Name | Propane |
Butane |
Pentane |
| Boiling Point | -42°C |
0°C |
36°C |
| Structural Formula | 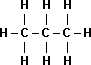 |
 |
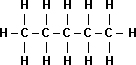 |
| Surface Charges |
|
Toggle Transparency |
|
 |
|||