Sodium Chloride - Ionic Bonds (1350 °C mp) |
Diamond - Covalent Bonds |
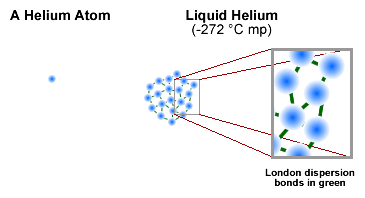
Some substances use only weak van der Waals bonds (London
dispersion).

Sodium Chloride - Ionic Bonds (1350 °C mp) |
Diamond - Covalent Bonds |

