Electron Configurations
- Although the original periodic table was arranged by properties
of the elements, Mendeleev didn't realize that it was the underlying structure
of the atoms that gave elements those properties. Today's table is based strictly
on the underlying structure. It looks very similar to the early tables, but
not exactly.
- So, to better understand the periodic table the properties of substances
we will need to explore the structure of the atoms.
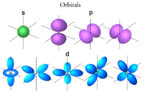
- An atom is made of a nucleus of protons and neutrons, and an
outer region containing electrons in orbitals (s,p,d, or f type).
- Each electron has a specific amount of energy associated with the orbital
in which it is found.
- Film: Orbitals
- The outer region of the nucleus is 10,000 times the size of the nucleus,
so the nucleus is buried deep inside the atom.
- Because the nucleus is tucked away beneath the electrons, it is the
electrons that give an atom its properties.
- Specifically, the outermost or valence electrons will primarily
determine how atoms interact with each other.
- Energy Levels and Electron Filling Order
- There are primary energy levels, and sublevels within each
primary level.
- Each row or period in the periodic table is considered to be the start
of a primary energy level.

- Each different type of orbital in a primary energy level is a sublevel.

- Each orbital can only hold
 two electrons and they must have opposite spin. This is called the Pauli
exclusion principle.
two electrons and they must have opposite spin. This is called the Pauli
exclusion principle.
- Electrons will fill up lowest energy orbitals first.
- The lower energy sublevels of one primary energy level can overlap with
the upper energy sublevels of another primary energy level. This can result
in orbitals of a higher principle energy level filling before the
orbitals in a lower principle energy level.
- To completely describe the electron configuration for an atom you need
to specify how many electrons are in each orbital at each level. This
is done with a specific kind of notation.

- Electron Configuration Examples (click on links to see a graphical representation
from www.webelements.com)
- H
= 1s1
- He
= 1s2
- Li
= 1s22s1
- O
= 1s22s22p4
- Click
here to see an applet which will display the electron configuration
of any element.
- You can also use a shortcut in writing electron configurations by
putting the previous closest nobel gas in brackets indicating that
you start with the electron configuration for that element and add
to it. For example,
Br = 1s22s22p63s23p64s23d104p5
= [Ar]4s23d104p5.



 two electrons and they must have opposite spin. This is called the Pauli
exclusion principle.
two electrons and they must have opposite spin. This is called the Pauli
exclusion principle.