Avogadro's Law
- Some typical data from the lab you did exploring the relationship
between moles and volume is shown below:
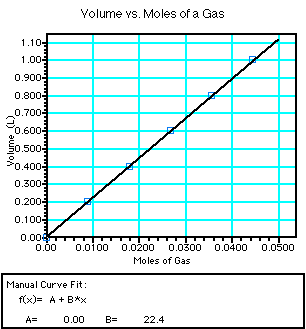
|
Moles of Gas
|
Volume (L)
|
|
0
|
0
|
|
0.0089
|
0.200
|
|
0.0179
|
0.400
|
|
0.0268
|
0.600
|
|
0.0357
|
0.800
|
|
0.0446
|
1.000
|
- Notice the simple relationship depicted by the graph. Is it directly proportional
or inversely proportional? If you don't know look at our other examples.
- Let's derive Avogadro's Law:
f(x) = B*x or
y = constant*x or
V= constant*n or
V/n = constant so
V1/n1 = V2/n2
- Two assumptions about the experimental conditions must be made for this
relationship to be true. What are they? (What other factors can affect volume?)
- Unlike the other "constants" which were found during the discovery
of Boyle's Law and Charles' Law, the constant found above holds true for all
gasses under the same temperature and pressure conditions.
- Avogadro did many experiments in which he chemically combined various volumes
of different gasses. They always combined in simple whole number ratios. This
lead Avogadro to conclude that equal volumes of gasses under the same temperature
and pressure conditions contain the same number of gas molecules. Even
though he didn't know how to calculate moles his experiments verified the
relationship we see above. Regardless of what gas is used, the same ratio
of volume to moles is always found: 22.4 L/mol at standard temperature (0°C)
and standard pressure (1 atm). This is known as the Standard Molar Volume
of a gas - 22.4L at STP.
