Human Sources of CO2
CO2
ADVANCED Strand Idea Sheet
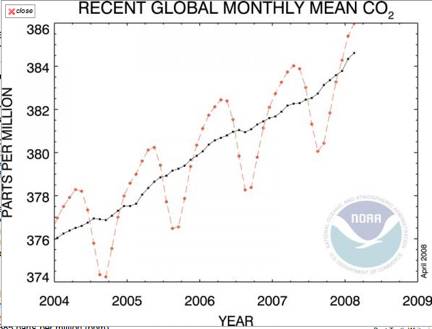
Carbon dioxide makes up only a tiny fraction of the Earth's atmosphere. But CO2 is going up steadily, in 2005 registering 379.1 parts per million (ppm), up from and 377.1 in 2004 and 350 ppm in 1990. In the 1850s, before the age of industrialization, the concentration was only about 275 ppm.
Sources: The primary cause of this increase has been the burning of coal, oil, and natural gas (fossil fuels - which contributes about 5.6 x 1015 tons (5.6 gigatons) of carbon to the atmosphere each year. Much of this is used in the production of electricity.
The rate of increase, even more worrisome, is increasing.
Iron, steel and cement manufacturing also releases a lot of CO2.
Deforestation, another large source of carbon to the atmosphere, contributes only between 0.5 and 2.0 gigatons by comparison.
Possible Investigations
Monitor CO2 levels in
areas where CO2 is being
produced.
Power plants, highways, or factories may produce patterns of CO2. How might you study these patterns?
¥ What are the human sources of CO2 at your study site or in your area? Can you estimate the quantity of CO2 that they produce?
Consider various sources of CO2.
How much CO2 does a car produce under various conditions?
Can you use lab tests to estimate the amount of CO2 emitted by fireplaces or factories?
What about first burning wood or coal in a lab?
Can you identify and study who are the large consumers of fossil fuels in your area?
How do they get rid of the products of combustion? Has this caused elevated levels of CO2 in the building or nearby?
VERY ADVANCED:
¥ How is CO2 dispersed from a known source? How does distance, terrain, or weather affect the dispersion of CO2?
¥ Smokestacks deliver CO2 and the other products of combustion up to a higher level in the atmosphere. Are these gases "fully mixed" with the atmosphere by the time they reach ground level?
¥ What is the quantity of CO2 that is "locked up" in the life at
your study site? How could you estimate this amount? When this life dies, is
there any difference in the amount of CO2 returned to the atmosphere