
Solutions and Dissolving
When solid materials are added to water, several changes can occur. Salt and sugar seem to disappear in the water. Rocks and gravel do not change. Particles remain visible, and may color the water. What is going on? |
Why We Care:
Our fresh water (and ocean water too, of course) typically contains dissolved minerals from soil, agricultural products such as fertilizer, bacteria and more, and salts from road treatment. Industrial byproducts find their way into our waters, sadly, too. |
What happens when something dissolves?
To determine which substances are soluble, we first need to understand the process of dissolving:
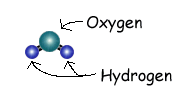 You probably remember that a water molecule is very small, with a single Oxygen and two Hydrogens. You probably remember that a water molecule is very small, with a single Oxygen and two Hydrogens.
The electrons of Hydrogen are pulled towards the Oxygen, making the two Hydrogen atoms more positive and the Oxygen more negative. (This attraction iis called a Hydrogen Bond.) So the whole molecule has a set of charges. These charges help keep one water molecule attracted to another.
The same charges mean that they can be very attracted to some substances when they are places in water. A tug of war often starts.
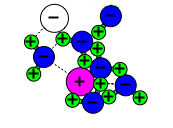 Let’s use the common table salt sodium chloride (NaCl) as an example. NaCl is a compound made of one sodium ion (Na+) and one chloride ion (Cl-) held together by an ionic bond resulting from the attraction between positive and negative ions. Several NaCl molecules bond together to form salt crystals. Let’s use the common table salt sodium chloride (NaCl) as an example. NaCl is a compound made of one sodium ion (Na+) and one chloride ion (Cl-) held together by an ionic bond resulting from the attraction between positive and negative ions. Several NaCl molecules bond together to form salt crystals.
Salt dissolves in water because water molecules have one negative end and one positive end.
See the model http://mw2.concord.org/public/student/solution/dissolve.html (An applet in browser) and http://mw2.concord.org/public/student/solution/watershell.html.
When NaCl is mixed with water, the strong polar ends of the water molecules attract the positive sodium ions and the negative chloride ions, pulling them apart.
Each ion becomes surrounded by water molecules attracted to it. This is why the salt seems to vanish in the water. The sodium chloride molecules have been split into ions, or dissolved, by the water molecules. Salt and other substances that dissolve in water are said to be “water soluble.” |
What does not dissolve?
 Gold, silver, and soil particles are examples of substances that do not dissolve in water. These substances contain molecules held together by strong, covalent bonds in which atoms share electrons. The positive and negative poles of the water molecules are not strong enough to pull apart a covalent bond, so they cannot surround the particles. Gold, silver, and soil particles are examples of substances that do not dissolve in water. These substances contain molecules held together by strong, covalent bonds in which atoms share electrons. The positive and negative poles of the water molecules are not strong enough to pull apart a covalent bond, so they cannot surround the particles.
There are exceptions to most rules! Some substances are held together by covalent bonds but have properties similar to ionic compounds. For example, sugar molecules are large, complex, and made of several types of atoms held together by covalent bonds. The bonds between the atoms have small charges on them. As these charges are sufficient to attract water molecules, sugar is soluble in water.
|
What is a solution?
Substances dissolved in a liquid form a solution. This means that the atoms of the dissolved substance are between the molecules of the liquid and cannot be separated by filtration. If you pour a solution of salt and water through filter paper, both the water and the salt will pass through the paper. In this example, the dissolved salt is called the solute, and the water that contains it is called the solvent. Liquids other than water also act as solvents for some substances. |
See dissolving in action!
Water: Getting to know the Water Molecule
http://molo.concord.org/database/activities/140.html
Dissolving: http://molo.concord.org/database/activities/237.html
Ionic Compounds: http://molo.concord.org/database/activities/54.html |
Back to Global Life Index Life Index
Back to Global Lab Water Index |

 You probably remember that a water molecule is very small, with a single Oxygen and two Hydrogens.
You probably remember that a water molecule is very small, with a single Oxygen and two Hydrogens.  Let’s use the common table salt sodium chloride (NaCl) as an example. NaCl is a compound made of one sodium ion (Na+) and one chloride ion (Cl-) held together by an ionic bond resulting from the attraction between positive and negative ions. Several NaCl molecules bond together to form salt crystals.
Let’s use the common table salt sodium chloride (NaCl) as an example. NaCl is a compound made of one sodium ion (Na+) and one chloride ion (Cl-) held together by an ionic bond resulting from the attraction between positive and negative ions. Several NaCl molecules bond together to form salt crystals.  Gold, silver, and soil particles are examples of substances that do not dissolve in water. These substances contain molecules held together by strong, covalent bonds in which atoms share electrons. The positive and negative poles of the water molecules are not strong enough to pull apart a covalent bond, so they cannot surround the particles.
Gold, silver, and soil particles are examples of substances that do not dissolve in water. These substances contain molecules held together by strong, covalent bonds in which atoms share electrons. The positive and negative poles of the water molecules are not strong enough to pull apart a covalent bond, so they cannot surround the particles.