- Strong bonds form between atoms when they share or transfer electrons.
- Depending on how even or uneven the sharing is between the atoms several deferent kinds of strong bonds can form.
- The way to determine if the atoms will share their electrons evenly or unevenly is to examine the electronegativity of each atom.
- Electronegativity is how strongly an atom attracts electrons to itself when bonded with another atom.
- The illustration below shows that atoms in the upper right corner of
the periodic table tend to attract electrons very strongly when bonded,
while the atoms in the lower left corner don't attract electrons to themselves
very well. (Except under unusual conditions, the noble gasses don't usually
form bonds, so electronegativity has no meaning for atoms which are not
bonded to other atoms.)
or if you prefer sphere sizes to represent the electronegativity:
- When two atoms are bonded together there are three basic ways to pair
them up:
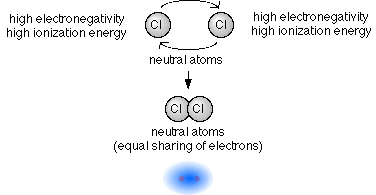
- Two atoms with the same electronegativity,
either both high or both low.
- This will cause the electrons that are shared in the bond to be evenly shared between the atoms.
- When atoms share electrons evenly between each other the bond
formed is called a non-polar covalent
bond.
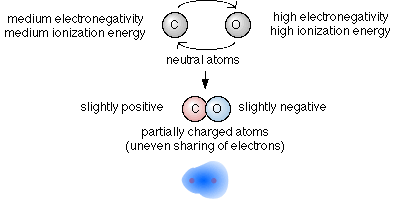
- One atom with a somewhat higher electronegativity
than the other.
- This will cause the electrons to be shared unevenly, such that the shared electrons will spend more time on average closer to the atom that has the higher electronegativity.
- When atoms share electrons unevenly but not very unevenly the
bond formed is called a polar covalent
bond.
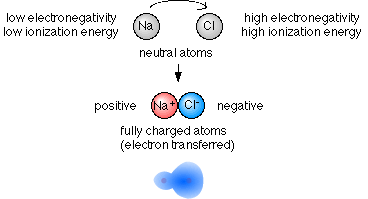
- One atom's electronegativity is much higher
than the other atom.
- In extreme cases the electrons in the bond spend so much time closer to the atom with high electronegativity that the shared electrons are considered to be transferred to that atom. The "sharing" is so uneven that one atom basically "takes" one or more electrons from the other atom.
- When the electrons being "shared" are so unevenly
distributed between the atoms the bond that is formed is called
an ionic bond.
- Two atoms with the same electronegativity,
either both high or both low.
- Non-polar covalent bonds
- If the difference in the electronegativity between the two bonded atoms is less than 0.5 then the bond formed is considered to be non-polar covalent.
- Each atom attracts the other atom's electrons about equally so that the electrons spend equal amounts of time near each atom.
- Overall, both atoms will be neutral, having the same charge.
- Polar covalent bonds
- If the difference in the electronegativity between the two bonded atoms is between 0.5 and 2.1, then the bond formed is considered to be polar covalent.
- One atom attracts the other atom's electrons better, so the electrons stay closer (on average) to that atom. This causes an imbalance of electric charge within the bond between the two atoms.
- The atom that pulls the negative electrons better toward itself
will be slightly negative and the other atom will be slightly positive.
Non-Polar
Polar
(H – H)
Electronegativity
Difference = 0.0
(C – O)
Electronegativity
Difference = 0.89
(H – F)
Electronegativity
Difference = 1.88Show molecular surface.
Show charge balance.
Show molecular surface.
Show charge balance.
Show molecular surface.
Show charge balance.

- If the difference in the electronegativity between the two bonded atoms is greater than 2.1, then the bond is considered to be ionic.
- Because one atom pulls the other atom's electrons so well toward itself, there is a great imbalance of electric charge. If for some reason the bond between the atoms is broken, the atom with the higher electronegativity will actually keep the electron for itself.
- In this case the atoms with the higher electronegativity will be fully negative (due to the "gaining" of an electron) while the other atom is fully positive (due to its virtual loss of an electron).
- Only the extreme cases are very clear. Very small differences
in electronegativity result in non-polar covalent bonds, and very large
differences in electronegativity result in ionic bonds. All other bonds
are somewhere in-between.
Type of BondDifference in ElectronegativityNon-Polar Covalentless than 0.5Polar Covalentbetween 0.5 and 2.1Ionicgreater than 2.1
- What kind of bond will form between the following atom pairs: