Niels Bohr (1885 - 1962)

|
Current Atomic Model 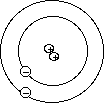
|
- One of the possible models proposed by Rutheford after completion
of his gold foil experiment stated that electrons orbit the nucleus like planets
orbit the sun. However, there were problems with this idea. For reasons beyond
the scope of this course, electrons would emit energy as they rotated around
the nucleus, eventually spiraling into the nucleus.
- Bohr proposed that electrons are orbiting, but that they can only orbit
at specific predefined distances from the nucleus. Electrons could exist in
one orbit or another, but nowhere in-between.
- He also proposed that electrons had very specific energies associated with
each orbit. Electrons that were close to the nucleus had lower energies than
ones orbiting at a distance. The energy of an electron was fixed or quantized
into discrete values based upon its current orbit.
- Bohr proposed his quantum mechanical model of the atom in 1913.

