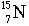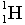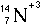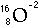Symbolic Representation of Atoms
- Atomic Symbols
- The subscript number is called the atomic number = number of
protons.
- The superscript number is called the mass number = protons+neutrons
- The number of protons defines the element. All carbon atoms have 6 protons.
All nitrogen atoms have 7 protons.
- Atoms of the same element can, however, have different masses. Each unique
form of an element has a specific number of protons and neutrons.
- Each form of carbon above would be called an isotope of carbon. Sometimes
they are referred to as Carbon-14 or Carbon-12. All isotopes of carbon have
the same number of protons, but their number of neutrons can differ.
- Atomic mass is the weighted average of the isotopes of a particular element.
For example ~99% of carbon is Carbon-12 and ~1% is Carbon-14. In any sample
of carbon on earth you will generally find this ratio. When you use a sample
of carbon it contains both isotopes, so you need a way of determining the
average atomic mass. Calculate the average atomic mass based on the abundances
of the isotopes listed above. It should match the atomic mass listed on the
periodic table - 12.01.
- Atoms of course are made of one other elementary particle - the electron.
Neutral atoms would exactly balance positive and negative charge. Each proton
carries a charge of +1 and each electron carries a charge of -1. Any atom
that is not neutral is called an ion.
| |
Protons
|
Neutrons
|
Electrons
|
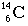 |
6
|
8
|
|
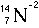 |
|
|
|
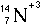 |
|
|
|
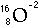 |
|
|
|
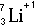 |
|
|
|
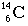 68
68