The Modern Atom (1950's - present)
Current Model of the Atom

Current Model of the Atom

 |
 |
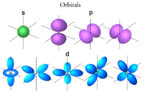 model consistent with the spectroscopic data that show electrons can only
exist in specific discrete energy levels. Click on the illustration to the
right to see cartoon images of these orbitals.
model consistent with the spectroscopic data that show electrons can only
exist in specific discrete energy levels. Click on the illustration to the
right to see cartoon images of these orbitals.