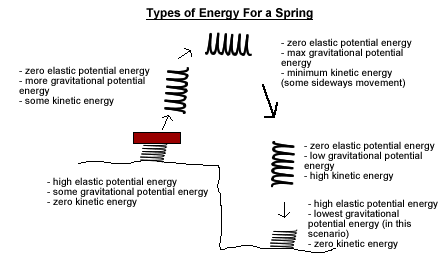
- Elastic Potential Energy
- Anything that can act like a spring or a rubber band can have elastic potential energy.
- Let's take the rubber band for example. To stretch the rubber band you have to use energy. That energy has now been turned into elastic potential energy. To get that energy back, just let go of the rubber band and its potential energy is converted primarily into kinetic energy.
- Springs work the same way, but you can either stretch or compress them. Wind-up watches store potential energy in an internal spring when you wind them and slowly use this energy to power the watch.
- Gravitational Potential Energy
- There is a constant attractive force between the Earth and everything surrounding it, due to gravity.
- To lift something off the ground it takes energy, so just by lifting an object, that object now has higher gravitational potential energy.
- Gravitational potential energy is typically converted into kinetic energy (an object falling) before it is converted into any other type of energy.
- Hydroelectric power is generated this way. As the water falls, it
turns a turbine, which pushes electrons around, creating an electric
current.
- Chemical Potential Energy
- A chemical bond can be thought of as an attractive force between atoms.
- Because of this, atoms and molecules can have chemical potential energy.
- Anytime two atoms form a strong covalent or ionic bond or two molecules form a weak van der Waals bond, energy is released, usually in the form of heat and light.
- The amount of energy in a bond is somewhat counterintuitive - the
stronger or more stable the bond, the less potential
energy there is between the bonded atoms.
Strong bonds have low potential energy and weak bonds have high potential energy.
- Lot's of heat and/or light energy is released when very strong bonds
form, because much of the potential energy is converted to heat and/or
light energy. The reverse is true for breaking chemical bonds. It
takes more energy to break a strong bond than a weak bond. The breaking
of a bond requires the absorption of heat and/or light energy.
|
Comparison of High
and Low Chemical Potential Energy for Various Bonds |
||||
| weak van der waals bond formation | strong covalent bond formation | strong covalent bond formation | breaking of weaker covalent bonds to form stronger covalent bonds | |
| higher chemical potential energy |  |
|||
| lower chemical potential energy | ||||
| The difference between the high and low is small because of the weak bond formation. | The amount of chemical potential energy released above is much greater than what is released during the formation of weak van der Waals bonds during condensing or freezing. | |||
previous page
chemsite homepage