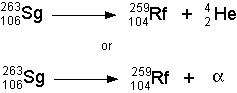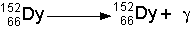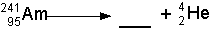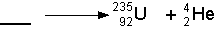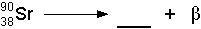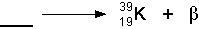Radioactive Decay
- Some isotopes are very stable, never spontaneously changing at
all. However, some isotopes can spontaneous go through a transformation which
causes them to emit radiation and usually change from one nuclear structure
to another, transmuting the atom from one element to another.
- Certain isotopes are radioactive because their nuclei are unstable. There
has to be a balance between neutrons and protons in the nucleus. The larger
the difference the more unstable the nucleus becomes. Also, as nuclei get
larger they also become unstable, so that all elements with an atomic number
greater than 89 are naturally radioactive.
- When the nucleus breaks down in some way to become more stable, we call
this a "decay". When atoms decay they give off radiation. Here we
will explore several of the most common ways an atom can decay.
- Alpha Decay
- If a nucleus ejects an a glob of two protons and two neutrons,
then it has just emitted an alpha particle. See the illustration below:
- The symbolic representation of the above decay would be:
- Because alpha particles are so big compared with other emitted radioactive
particles they can't penetrate very far into matter, but they do a great
deal of damage where they are absorbed. Alpha particles can only travel
a few centimeters through air and can be blocked by a piece of paper.
However, if you ingested or inhaled even a microscopic dust grain of plutonium
then there is a very high chance cancerous growth will occur wherever
this grain of plutonium (an alpha emitter) gets lodged in your system.
- Practice. Try filling in the blanks below. Click on each one to see
the answer:
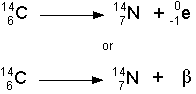
- Beta Decay
- If a nucleus ejects an electron, then it has just emitted a
beta particle. You might ask how a nucleus can eject an electron because
electrons are not in the nucleus. However, this happens through the transformation
of a neutron into a proton. If a neutron becomes a proton, then an electron
(beta particle) is ejected at high speed. Also, a neutrino is ejected
as well, but neutrinos almost never interact with matter, so we don't
worry about their biological effects. See the illustration below:
- The symbolic representation of the above decay would be:
- Beta particles are much smaller and can easily go through a piece of
paper. They can travel several meters through air and can be stopped by
a thin sheet of lead.
- Practice. Try filling in the blanks below. Click on each one to see
the answer:
- Gamma Decay
- Sometimes a nucleus is in an excited state, and to become stable
it only needs to emit some energy. When pure energy is emitted with no
material change in the nucleus, a photon of gamma radiation is emitted.
See the illustration below:
- The symbolic representation of the above decay would be:
- Gamma rays have no mass at all and can penetrate matter extremely well.
They can travel through many kilometers of air, but are blocked by thick
sheets of lead.
- An excellent summary of all these concepts can be found at: http://www.darvill.clara.net/nucrad/types.htm