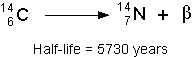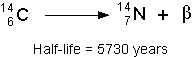Radioactive Dating
- You can use the fact that radioactive isotopes have a constant
half-life to determine the age of something containing radioactive material.
- Let's take Carbon-14 for example with a half-life of 5730 years. This naturally
occurring isotope decays via the emission of a beta particle as shown below:
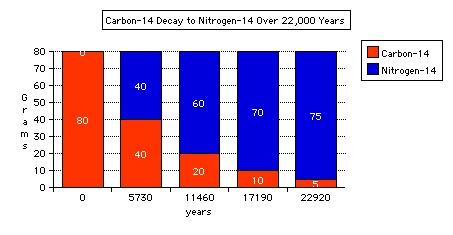
- If we know how many Carbon-14 atoms we start with, then we can measure how
many are left. If half of the original amount of Carbon-14 has decayed then,
5730 years have passed. See the illustration below:
- Radioactive Carbon-14 is constantly created in our atmosphere by cosmic
rays. Over long periods of time ration of Carbon-14 to Carbon-12 has remained
relatively constant.
- All living things constantly take in carbon, either as carbon dioxide (in
the case of plants), or as food in the form of vegetation or meat. Therefore,
while something is alive it will have the same ratio of Carbon-14 to Carbon-12
in its body as the surrounding environment. However, as soon as something
dies it stops replenishing its system with carbon so the amount of Carbon-14
becomes fixed at death. At this point, as the organic matter ages, the amount
of Carbon-14 present is less and less as it decays to Nitrogen-14.
- As you can see from the graph above after about 20000 years the % of Carbon-14
that remains is quite small, so carbon dating can only be used to date organic
material that is younger than 20-30 thousand years old.
- Other isotopes are used to date rock and other materials that may be far
older.