- The molar mass of a substance is defined as the mass of one mole of particles of that substance.
- A particle or unit of a substance is defined by its formula. For example:
-
Copper as a general substance looks like this ---> Its formula is Cu, so a mole of copper means a mole of these --> 
-
Oxygen as a general substance looks like this ---> Its formula is O2, so a mole of oxygen means a mole of these -->
-
Water as a general substance looks like this ---> Its formula is H2O, so a mole of water means a mole of these -->
-
Sodium Chloride as a general substance looks like this ---> Its formula is NaCl, so a mole of sodium chloride means a mole of these -->
(The molecular modles above were used with permission and created by C.H. Mak at Virginia Tech. University)
-
- Notice in the above examples that the formula of a substance defines the basic submit of that substance. When doing calculations we need to relate the mass of a substance to the mass of one mole of that substance. For monatomic elements this is easy. The mass of one mole is equivalent to the atomic mass in grams. For example, the mass of one mole of sodium is 22.99 g. This is more commonly referred to as the molar mass.
-
However, to calculate the molar mass of any other type of substance we use the formula to determine how many moles of atoms are contained in one mole of the substance. See examples below:
Copper has the formula Cu so one mole of copper has 1 mole of copper atoms. Its atomic mass is 63.55g. 1 x 63.55g = 63.55g
Oxygen has the formula O2, so one mole of oxygen has 2 moles of oxygen atoms. It's atomic mass is 32.00g. 2 x 16.00g = 32.00g Water has the formula H2O, so one mole of water has 2 moles of hydrogen atoms and 1 mole of oxygen atoms. It's atomic mass is 18.02g. 2 x 1.008g = 2.016g
1 x 16.00g = 16.00g18.02g Sodium chloride has the formula NaCl so one mole of sodium chloride has 1 mole of sodium atoms and 1 mole of chlorine atoms. It's atomic mass is 58.44g. 1 x 22.99g = 22.99g
1 x 35.54g = 35.54g58.44g
- What is the molar mass of calcium phosphate - Ca3(PO4)2?
- One way to identify compounds is to determine what percentage each element is by mass in a compound.
- See the example of water from the above table. The molar mass of sodium
chloride is 58.44g. 22.99g of this is sodium and 35.45g is chlorine. If
I want to calculate the percent composition of sodium chloride, I would
do the following:
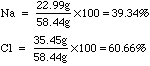
- What is the percent composition of calcium phosphate?
previous page
chemsite homepage