- Pulling forces from the intermolecular attractions.
- Pushing forces from the pressure applied on all surfaces of the liquid (from both the container and the gas above the liquid)
- It is an inherent property of a substance like density.
- Can be measured by sealing a liquid inside a container and measuring
the pressure created by the molecules which naturally evaporate at that
temperature.
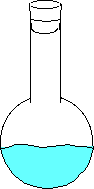
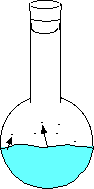
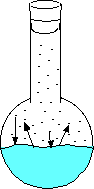
Here the water has just been added and no vapor has evaporated. Here the water begins to evaporate. The gaseous molecules are beginning to increase the pressure inside the flask. Eventually enough molecules are bouncing around such that the rate of evaporating molecules equals the rate of condensing molecules. This is when you can measure the equilibrium vapor pressure. - An increase in temperature will increase the vapor pressure.
Why?Vapor Pressure of Water at Various Temperatures Temperature (°C) Pressure (atm) 0 0.0060 20 0.0231 40 0.0728 60 0.1968 80 0.4675 100 1.0000 - An increase in intermolecular forces will decrease the vapor pressure. Why?