Ideal Gas Law
- With the Combined Gas Law, our only assumption was that the moles
remain constant. Now we have a way of incorporating even a change in the number
of gas moles using Avogadro's Law. See the derivation below:
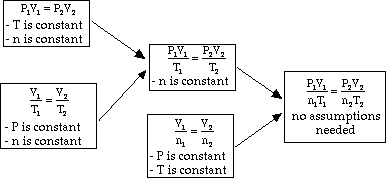
- In the case were all variables are incorporated (P,V, T, and n (moles))
the constant calculated here must be the same for all gasses under all pressure,
volume, temperature and mole size conditions. This special constant is called
the universal gas constant. If we use atm, L, K, and mols for our units,
the the value of the constant is:
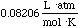 and is given the symbol R.
and is given the symbol R.
- So, if you take any gas and measure its pressure, volume, temperature, and
moles and calculate:
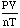 the answer will be
the answer will be 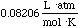 or
R.
or
R.
- We can, therefore create the following equation:
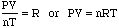
- This is known as the Ideal Gas Equation (PV=nRT) and can be used
to calculate pressure, volume, temperature or mole quantity of a gas.
- When to use the above equations:
- Use
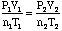 when
the problem is posed in such a way that the environmental conditions of
the gas are changing from one P,V,T or n to another.
when
the problem is posed in such a way that the environmental conditions of
the gas are changing from one P,V,T or n to another.
- Use PV=nRT when the problem asks you to calculate some quantity of a
gas that is under one set of conditions, not changing in any way.

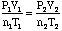 when
the problem is posed in such a way that the environmental conditions of
the gas are changing from one P,V,T or n to another.
when
the problem is posed in such a way that the environmental conditions of
the gas are changing from one P,V,T or n to another.