- Gas pressure is related to the number of impacts per square inch on the surface of its container.
- As you increase the volume, you spread out the gas particles, causing fewer impacts per square inch, and lowering the gas pressure.
- In a way, the pressure was related to the concentration of gas molecules
in empty space in the same way solution concentration is related to how
much solvent is used to dissolve a given amount of solute. See the illustrative
comparison below:
For Gasses 

low volume = high pressure/concentration high volume = low pressure/concentration For Solutions 

low volume = high concentration high volume = low concentration
- Notice that if we rearrange the Molarity equation we get:
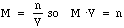
- Notice this shows that Molarity times the Volume give you the number of moles. Just like in our gas example, where the number of moles of gas molecules remains constant when we change the volume, the number of moles of solute remains constant if we add more solvent.
- So, we know that if we dilute a concentrated solution (or make a solution
more concentrated by evaporating solvent) that the moles of solute remains
constant, allowing us to come up with the following formula:
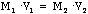
- This should look very familiar - just like Boyle's law.
- NOTE: It only makes sense to use this formula for dilutions!
- If you were given 200.0 mL of a 12.0 M solution and were asked to dilute this to 4.0 M, what would be the final volume of your solution?
- Assume you had a large quantity of 5.0 M silver nitrate and you wanted to make 300.0 mL of 0.010 M solution. How would you do it? Click here for answer.