- The strong surface tension of water prevents it from getting into small spaces.
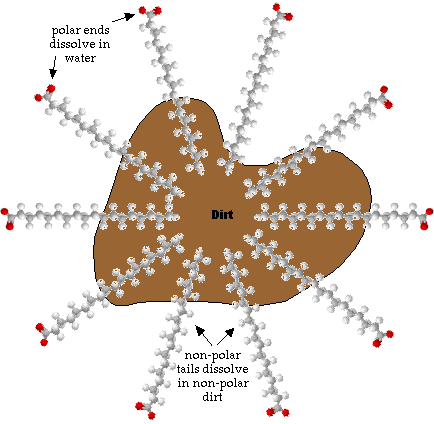
- Most dirt is non-polar so it doesn't dissolve well in polar water.
| Show molecular suface with electrostatic potential |
- Notice that one end is polar and the other end is non-polar. This make sodium sterate a kind of schizophrenic molecule. It can dissolve in both polar and non-polar substances.