| CH4 + | 3 CuO | ---> | 3 Cu + | 2 H2O + | CO | |
| basic | 1 molecule | 3 units | ---> | 3 atoms | 2 molecules | 1 molecule |
| x2 | 2 molecules | 6 units | ---> | 6 atoms | 4 molecules | 2 molecules |
| x12 | 12 molecules | 36 units | ---> | 36 atoms | 24 molecules | 12 molecules |
| 1 dozen | 3 dozen | ---> | 3 dozen | 2 dozen | 1 dozen | |
| x6.02e+23 | 6.02e+23 | 1.81e+24 | ---> | 1.81e+24 | 1.20e+24 | 6.02e+23 |
| 1 mole | 3 moles | ---> | 3 moles | 2 moles | 1 mole |
- Consider the following problem: Given the reaction below, how
many moles of aluminum oxide will you produce if you start with
5.40 mol of aluminum?
4 Al + 3 O2 --->2 Al2O3 -
First think about what the chemical equation states. The question asks about the relationship between aluminum oxide and aluminum. We can see from the reaction that if 4 moles of Al are used then 2 moles of Al2O3 will be produced. This ratio is fixed, so if we start with 5.40 moles of Al instead, how do we predict the amount of Al2O3 produced?
- To do this we must set up a ratio between the two substances asked about
in the question.
so5.40 x 4 Al + 3 O2 ---> 2 Al2O3
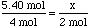
- By solving for x, you will determine the moles of aluminum oxide produced.
- So, the balanced chemical equation can be used to set up a MOLE RATIO between any two substances in the equation.
-
It is important to remember that you can only set up a