ABOUT WATER TEMPERATURESWater has two unique characteristics: it is a very poor conductor of heat and it has a great capacity to hold energy. In other words, iit takes a lot of energy to raise the temperature of water, but once the temperature is raised, the heat energy is dissipated very slowly . |
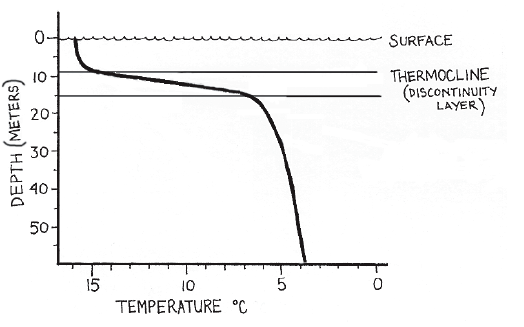
How much do Water Temperatures Change?When sunlight strikes the surface of water, it heats the top layers most readily. The sunlight is transmitted by the water only enough to heat lower levels a little bit. In the ocean, most of the light energy is absorbed in the top 50 centimeters. At 2 meters depth, 98% of the energy has been absorbed and transformed into heat. Since water has a high capacity to hold on to hea - once heated it stays hot for a time, seasonal changes in ocean temperatures lag about 2 months behind land temperatures. So in temperate climates, the ocean is much warmer for swimming in the fall than in the spring. This temperature lag is even greater in deeper layers of larger lakes and in oceans. The lag can stretch to as long as 5 months (Russell-Hunter, 1970). Heat is transferred to lower levels of lakes, ponds, and oceans largely by the circulation of the body of water. Water circulation is caused by the movement of water molecules between areas of different densities. Since water near the surface is less dense than water near the bottom, the water circulates, bringing the solar-heated water of the surface down into the body of water and heating the lower levels. The water near the surface and warmed by the Sun is less dense than water near the bottom, because density of water changes as the temperature of the water changes. The lower the temperature of water, the higher the density of that water—until around 4ºC. At Thermoclines
If the surface layers of a body of water are undisturbed by wind or tides, layers of water with different temperatures can lie on top of each other without mixing because differing densities. When the upper water layers warm in the summer months, they become separated from deep water by a transition zone known as a thermocline. In a thermocline, the temperature decreases rapidly with small increases in depth. This phenomenon linking temperature change with depth is called temperature stratification. Temperature stratification occurs in most bodies of water from small ponds to oceans, but thermoclines are primarily found in the larger bodies of water. Almost no exchange of water or solutes takes place across an established thermocline. For example, if rich river sediment is flowing into an ocean bay, this sediment will flow across the top of the thermocline and not move into deeper currents that might carry it elsewhere. Thermoclines occur in tropical, equatorial seas and lakes but never in polar seas. This absence of thermoclines near the poles is the result of water circulation patterns and low levels of sunlight. The solar energy entering polar waters is not enough to raise the surface temperature significantly. Without this warm top layer, a thermocline cannot form. Also the positions of land masses in the polar latitudes cause strong circumpolar ocean currents that would disrupt any thermocline formation. Thermoclines in lakes are considerably more stable than in oceans, because ocean tides and currents cause more mixing of the water. |
SEASONAL CHANGES IN TEMPERATURE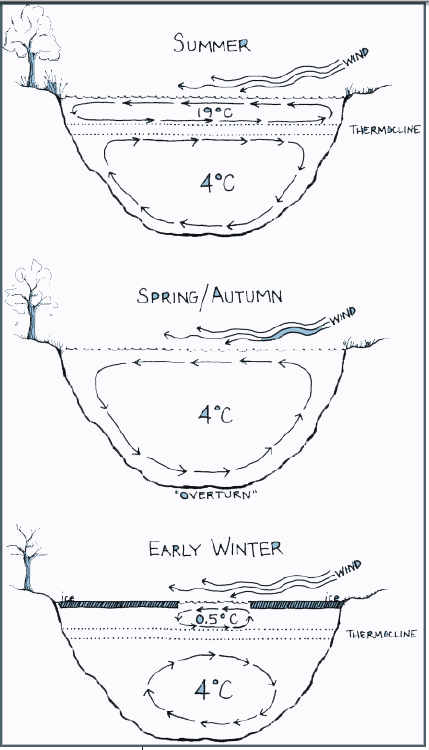
In lakes, mixing occurs for short periods of time in the spring and fall. During the summer, increased solar radiation and resulting higher air temperatures warm the surface layer. Wind stirs this upper layer and a “rolling barrel” effect is created with the layer below the thermocline. The thermocline ensures that a minimal amount of mixing occurs between the lower barrel and the upper barrel. Solar radiation levels and air temperatures decline in the fall, but water temperatures do not begin to decline until late fall. (Remember the lag time effect described above.) Thus, in late fall, the surface water temperature decreases and the temperatures of the two barrels approach each other. As this happens, the thermocline breaks down. This allows mixing of the upper and lower water systems. The air temperature continues to decline with the onset of winter, and the surface layer of the lake freezes. The water just below the ice can become supercooled, decreasing to below 4°C. This water is less dense than the water in deeper layers and once again a thermocline forms. With spring warming, the ice melts as the temperature rises and the thermocline is disrupted. The mixing brings nutrients locked in the lower “barrel” to the surface. This allows nutrients to enter various food webs and leads to the development of algal and microorganism blooms. Ocean upwellings are fundamentally the same as those described above for lakes. Seasonal thermoclines are disrupted when the temperatures of the deeper currents approach the temperatures of the surface currents. In oceans, mixing results in massive plankton blooms in the upwelling. The movement of the current carries the upwelling to a different location. For example, mixing on the Antarctic shelf fuels upwellings along the western coasts of both South America and Southern Africa. |
Return to Water Dissolves Index Return to Water Index |