Atomic Theory
- Handout: Atomic
Theory Note Packet
- Black Box Model

- Early Theories - 400 B.C.
- Common Greek theory was that all matter consisted of four
"elements" - earth, air, fire, and water.
- Democretus (460-360 BC)
- Alchemy, the process of changing base metals to gold, was the chief
form of experimentation from this time period until the the late 1600's.
- Robert Boyle (1622 - 1691)
- Antoine Lavoisier (1743 - 1797)
- Lab: Does
Mass Change During Chemical Reactions?
- Homework: Does Mass Change During
Chemical Reactions Lab Questions.
- Joseph Proust (1754 - 1826)
- Demo: The Big Bunsen Burner
- Lab: Adjusting
the Bunsen Burner
- Lab: Making
water the right way. Put loudest pop ratio on the board when done.
- Handout: Good
Graphing.
- Homework: Making
Water the Right Way Lab Questions

- John Dalton (1768 - 1828)
- Handout: Dalton's
Atomic Theory
- Homework: Put the five parts of Dalton's
atomic theory into your own words.
- William Crookes (1832 - 1919)
- Plum Pudding Model
- J.J. Thompson (1856-1940)
- Demo: Magnet with Crookes tube.
- Homework: What are cathode rays and
how do they behave differently than light rays?
- Hard nucleus model
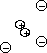
- Radioactivity
- Ernest Rutherford (1871 - 1937)
- Bohr Model of the atom
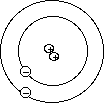
- Niels Bohr (1885 - 1962)
- Spectroscopy and Bohr's Model
- Electromagnetic Radiation (EMR)
- Production of EMR
- Homework: Electron
Jumping Worksheet; Read Light
Your Candy Article from the October '90 Edition of ChemMatters
- Emission and absorption spectroscopy
- Lab: Flame tests
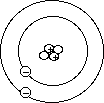
- Homework: Read
"Space 1998" article and underline each time were EMR
is used in some sort of way to learn more about distant objects.
- James Chadwick (1891-1974)
- The Modern Atom (1950's - present)
- Handout: Modern
View of the Proton (Download this and open it in an image editing
program to print.)
- Demo: Atom in a Box Software
- Computer Lab: Explore
the modern atomic orbitals.

- Video Clip: Seeing
Atoms

- Symbolic representation of atoms
- Homework: Atomic
Practic Sheet
- Handout: Review
Sheet





